Quality System Installation / Refinement
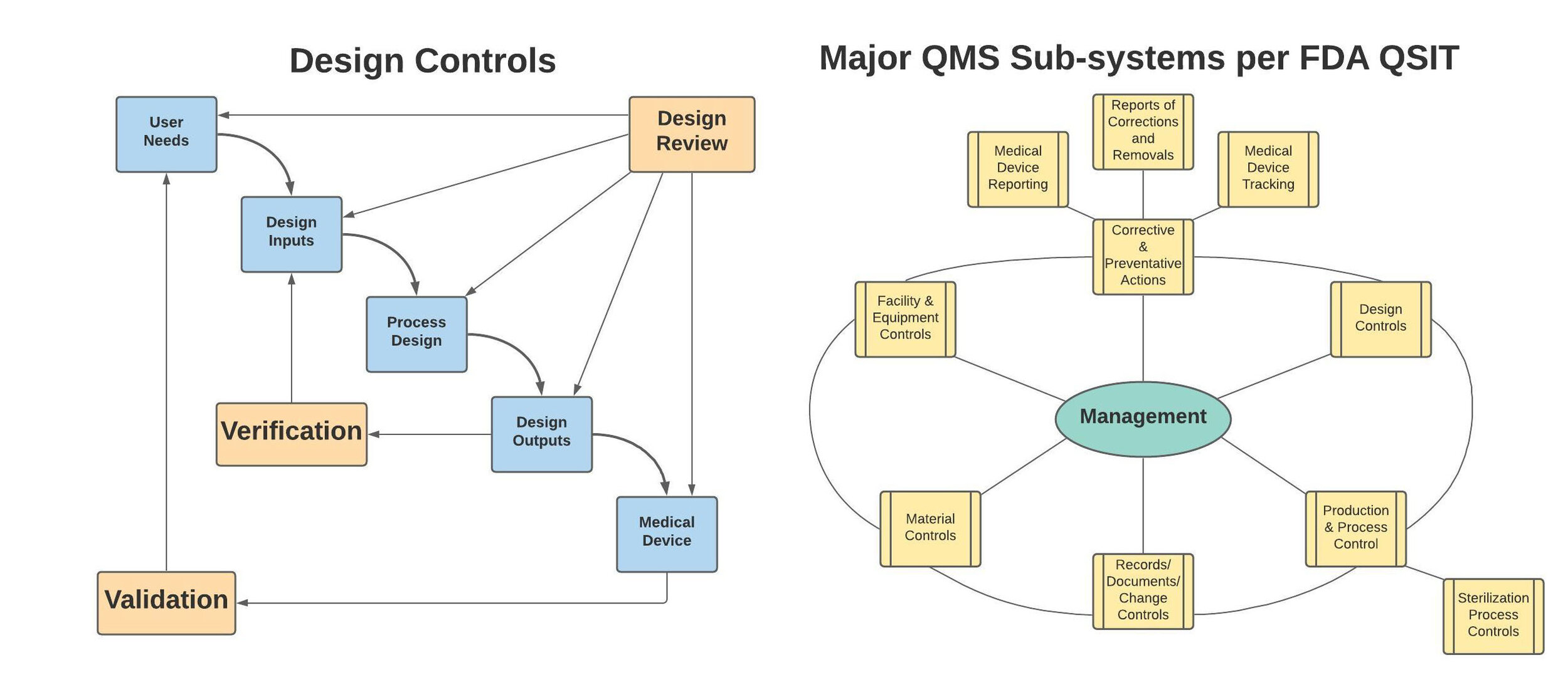
A robust quality system is essential to successfully manage any medical device company. We can help you install a QMS that is not only compliant to FDA 21 CFR part 820 and ISO 13485 requirements, but practical in its execution with respect to your specific business needs.
If you already have a QMS, but need help remediating one or more aspects, we can help with that too.