Our Approach
Every device problem is different, but the way we approach them is consistent.
We begin by breaking a problem down into the specific functional behaviors that matter—such as motion, force, stiffness, torsional response, or visual performance—rather than starting with a predefined test method. From there, we assemble a custom test solution by integrating only the tools and capabilities required to isolate and measure those behaviors.
We maintain a broad set of modular, highly integrated assets, including motion stages, force and torque sensing, vision systems, and custom fixturing. For a given application, a subset of these capabilities is selected and combined into a focused test configuration tailored to the specific question being asked. This modularity allows rapid adaptation from one application to the next without rebuilding systems from scratch.
Once data is generated, we apply custom internal data-processing scripts developed to match the test configuration and outputs. These scripts enable efficient analysis, visualization, and comparison of results, transforming raw measurements into actionable insight.
The result is a repeatable process that allows us to move quickly from problem definition to test execution to meaningful conclusions—using only the level of complexity required to answer the question at hand.
How this works in practice
Conceptually, this approach can be viewed as a set of integrated capabilities at our disposal—such as vision, motion control, force and torque measurement, and custom fixturing—from which a targeted subset is selected for each application. These selected elements are integrated into a custom test setup, supported by tailored data analysis, to generate detailed outputs that drive insight and problem resolution.
The application examples that follow illustrate how this approach is applied across design, process development, manufacturing sustaining, and product performance challenges.
We work under strict confidentiality with our clients and do not disclose client names or project-specific details. The examples below are presented at a high level to illustrate common application areas and approaches rather than individual engagements.
A broad set of integrated capabilities is selectively combined into a custom test configuration for each application, supported by tailored data analysis to generate actionable insight.
Design & Development Applications
In early development, reliance on finished-device build-and-test cycles often slows learning and limits meaningful design iteration. These cycles frequently identify that performance is not meeting expectations without clearly revealing which design or process variables are responsible. We shift learning earlier by isolating performance drivers through component- and subassembly-level testing.
Torque not meeting specification
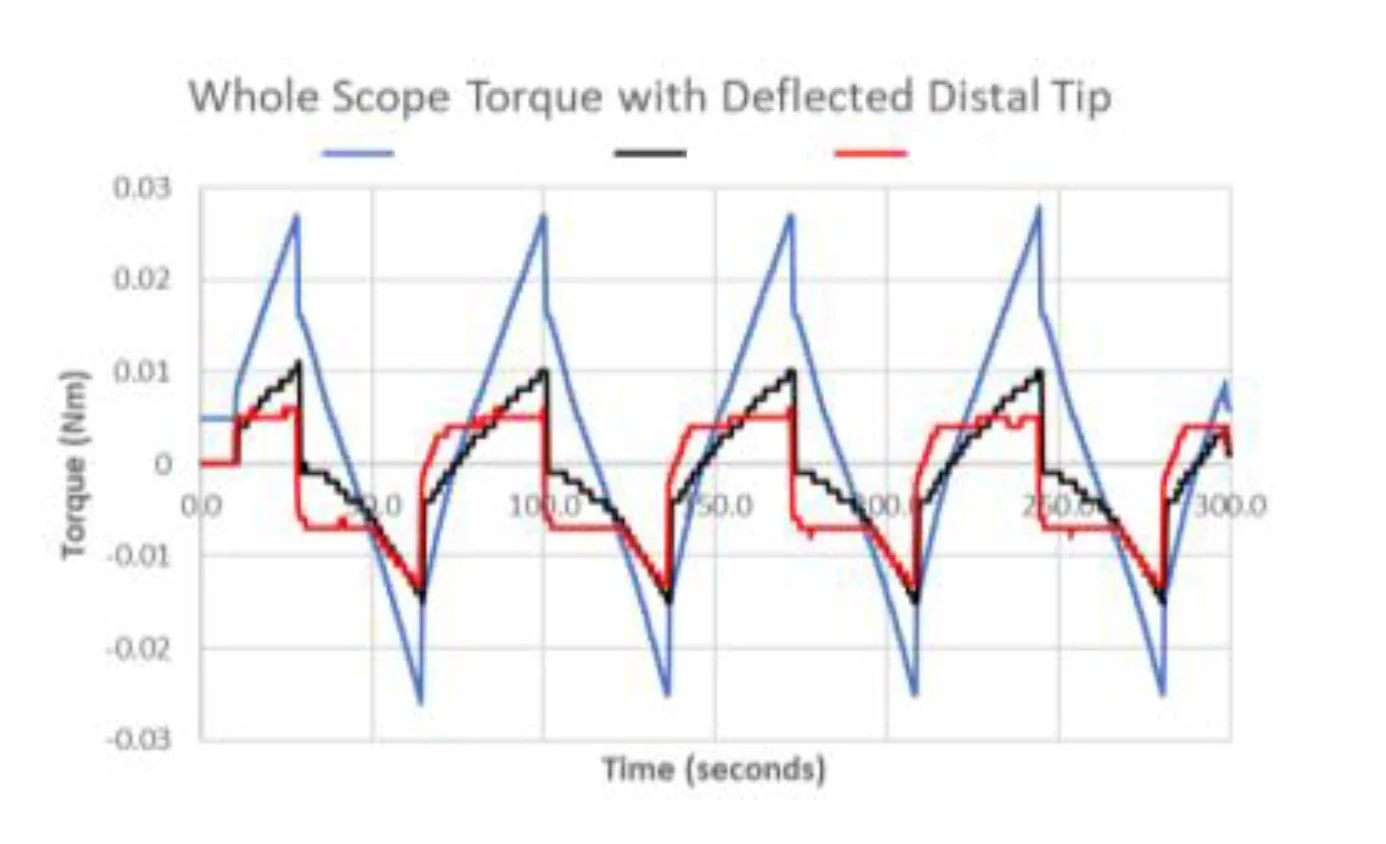
Example: For a complex articulating catheter, we evaluated multiple articulating link geometries and processing conditions at the subassembly level rather than relying on repeated finished-device builds. Testing focused on how local stiffness, joint compliance, and processing variation influenced articulation response and repeatability. By establishing quantitative relationships between link design, processing conditions, and functional output, we were able to predict finished-device articulation behavior with a small number of samples. This enabled faster iteration across multiple design options while reducing development time and cost.
Root Cause identified through LSM integrated testing
Design iterations & optimization through simplified component /sub assembly test model
Optimize link geometry of articulation shaft
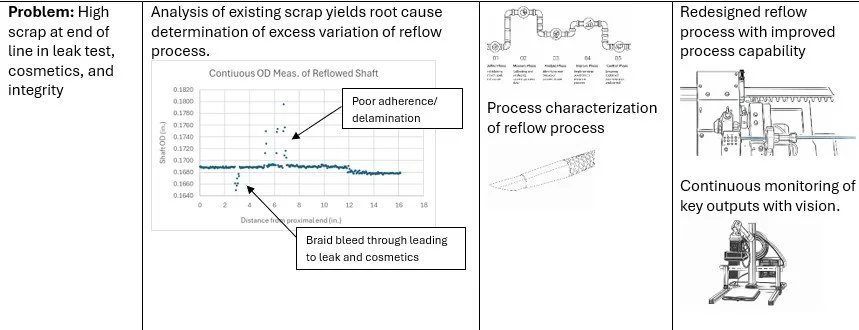
Optimize reflow of insertion tube
Design solution identified faster without need for full assembly builds.
Disclaimer: Examples are hypothetical based on real data independently procured by LSM from commercially release product and meant to represent the type of problems we are able to solve for clients.
Process Development & Characterization
As designs mature, teams often recognize opportunities to improve manufacturability or robustness through alternate designs or processing approaches, but these options are frequently not explored due to the perceived time, cost, and complexity of characterization. As a result, processes may enter validation with limited understanding of true operating windows, increasing the risk of late-stage rework or constrained manufacturing performance. We address this by focusing process characterization on a small number of high-value questions early, without relying on large builds or exhaustive studies.
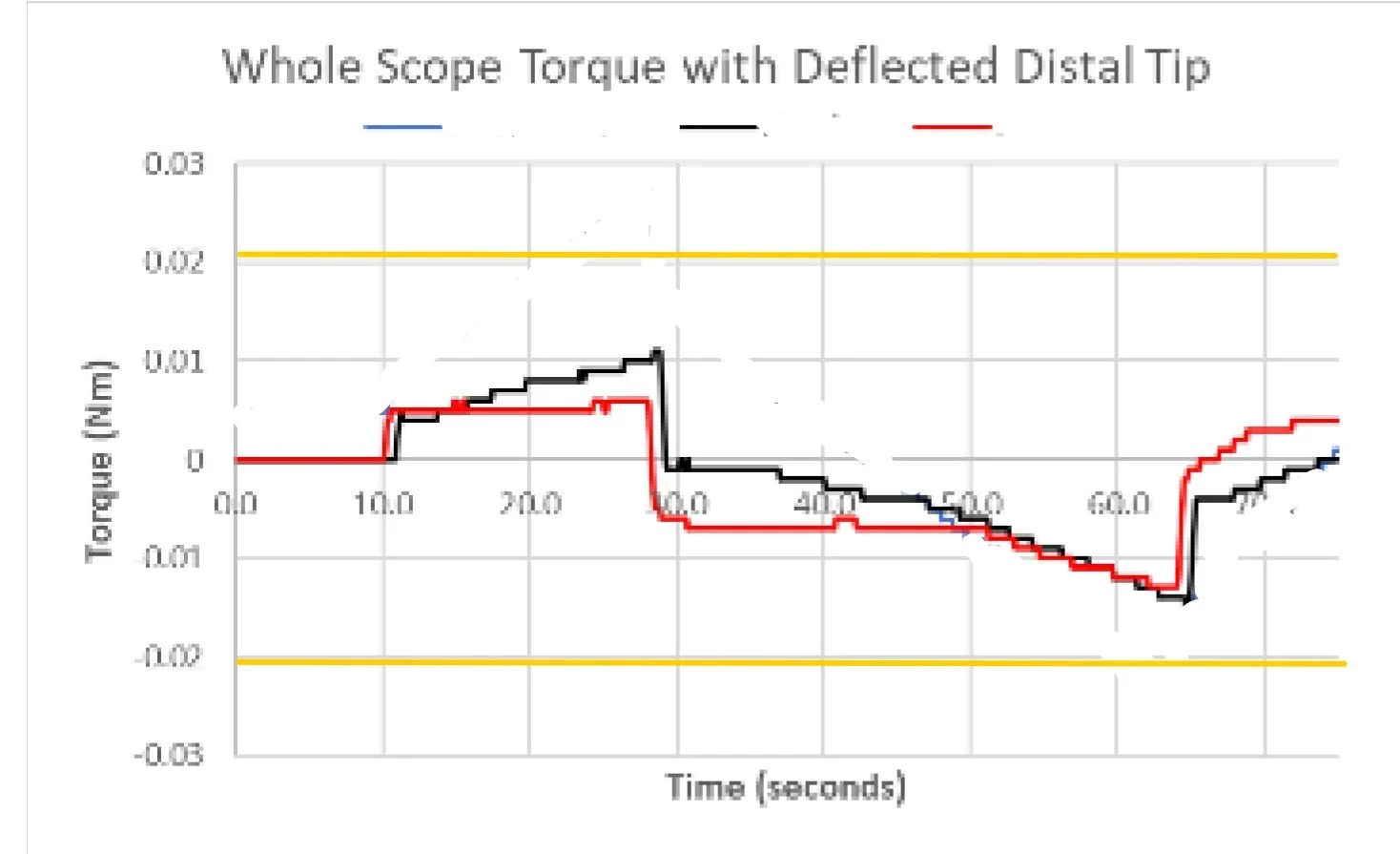
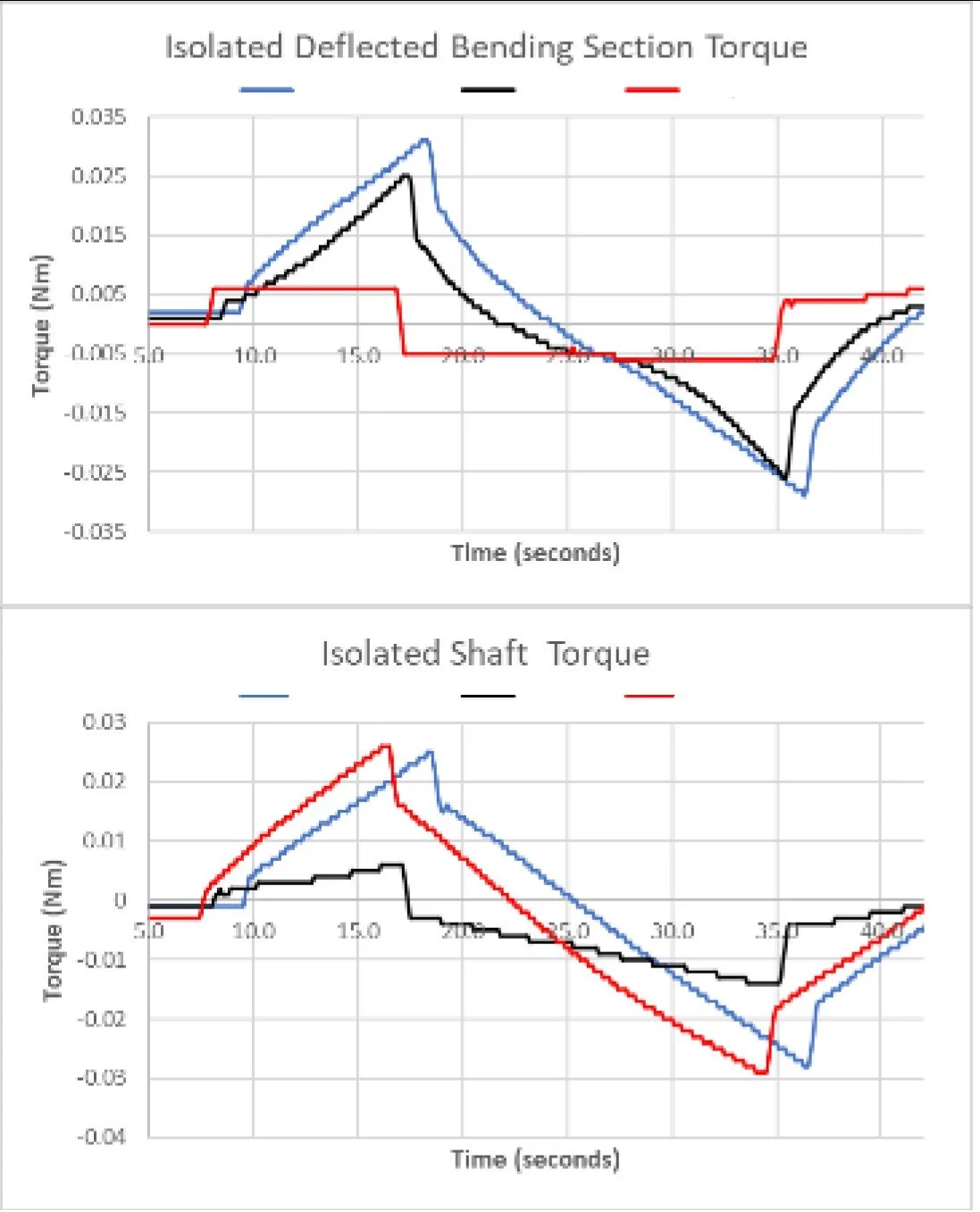
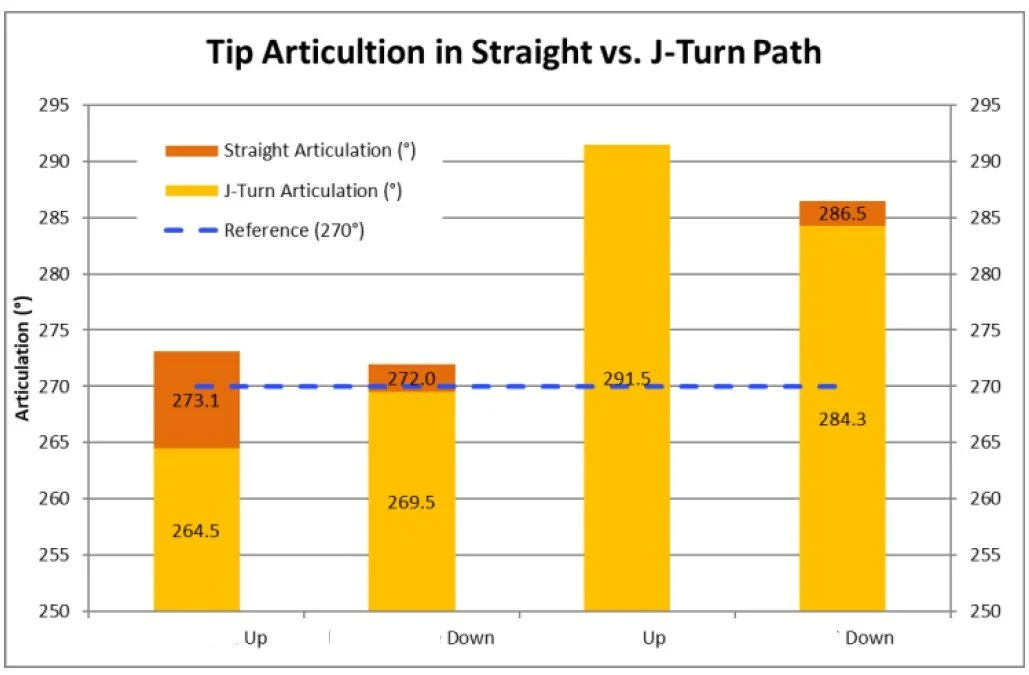
Reflow contribution to variation in shat stiffness and torsion
Example: For a complex multilayer laminated catheter, we established a rapid supply chain capable of producing controlled variations in materials, layer construction, and processing conditions within a short timeframe. Component- and subassembly-level testing was used to evaluate how specific design limits interacted with reflow and bonding processes to influence functional performance. This targeted characterization identified combinations that improved robustness and reduced reliance on adhesive bonding, enabling improved manufacturability prior to validation.
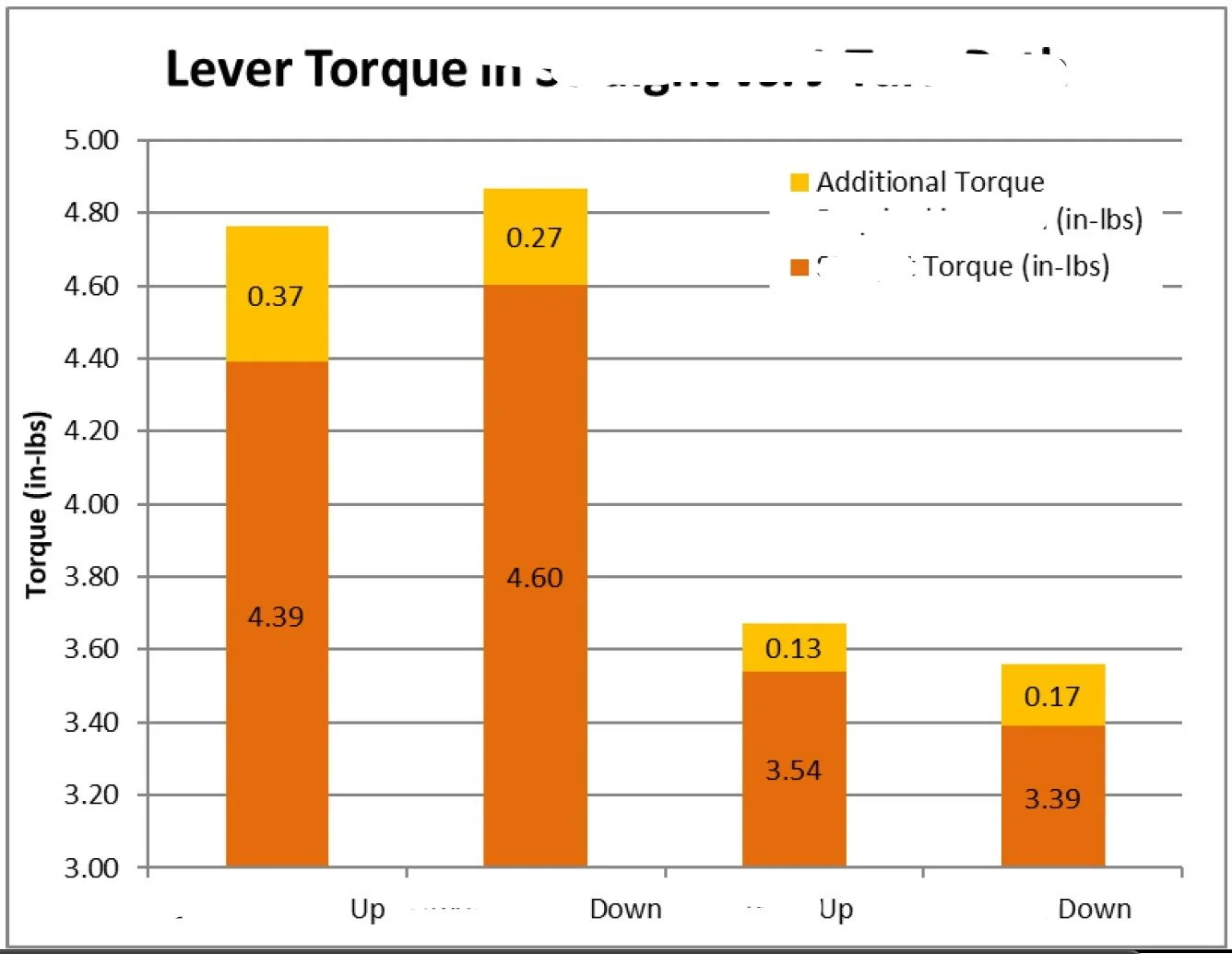
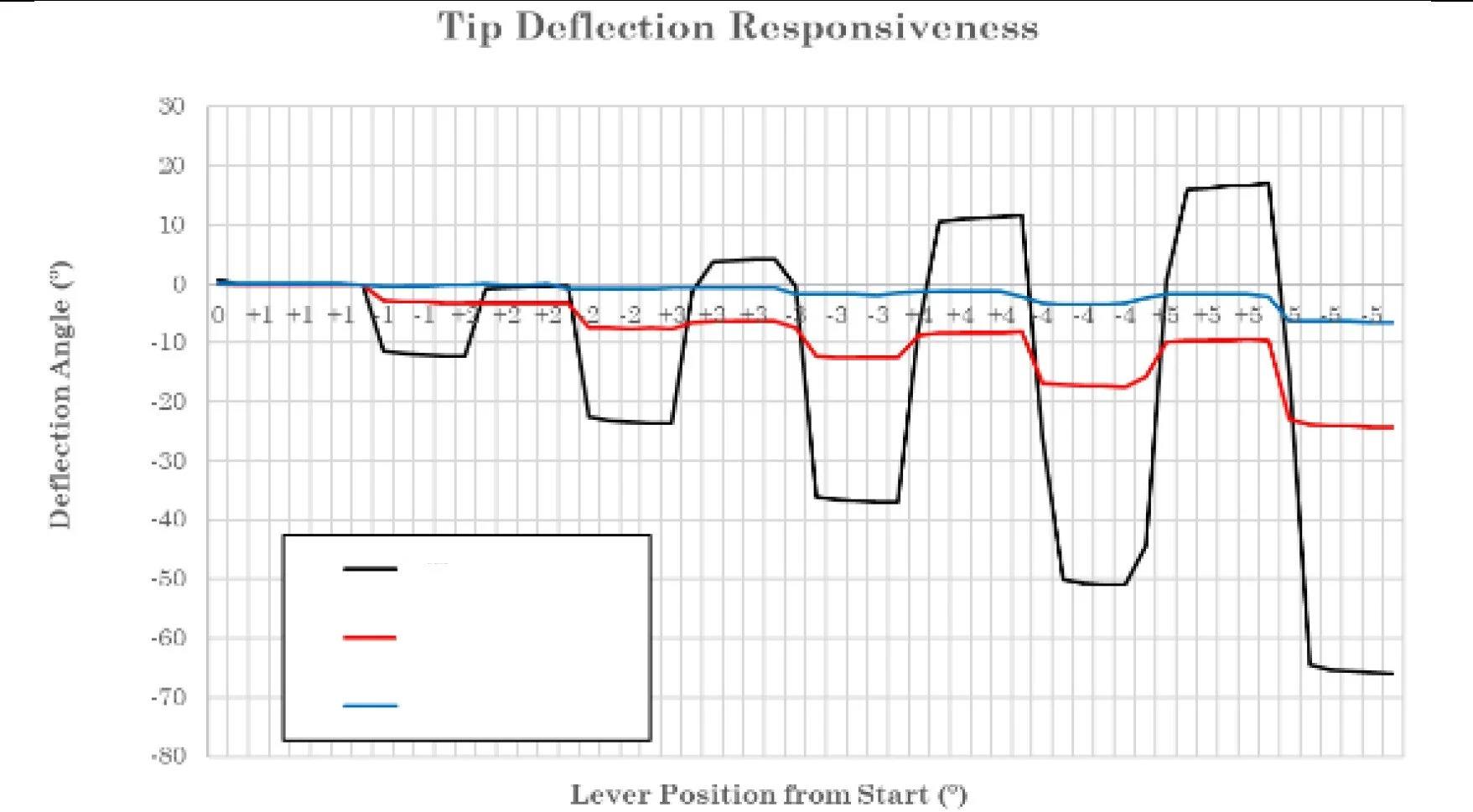
Effect of over tensioning of lever torque
Manufacturing Sustaining & Yield Improvement
In production, sustaining teams are often focused on keeping product flowing, leaving limited opportunity to investigate yield issues beyond short-term containment actions. Root cause frequently lies in subtle interactions between design, materials, and process steps that are difficult to isolate amid daily production demands. We support sustaining teams by acting as a dedicated parallel engineering resource, focused on targeted investigation of the factors that drive yield and cycle time.
Effect of over tensioning of lever torque
Manufacturing Sustaining & Yield Improvement
In production, sustaining teams are often focused on keeping product flowing, leaving limited opportunity to investigate yield issues beyond short-term containment actions. Root cause frequently lies in subtle interactions between design, materials, and process steps that are difficult to isolate amid daily production demands. We support sustaining teams by acting as a dedicated parallel engineering resource, focused on targeted investigation of the factors that drive yield and cycle time.
Effect of over tensioning of lever torque
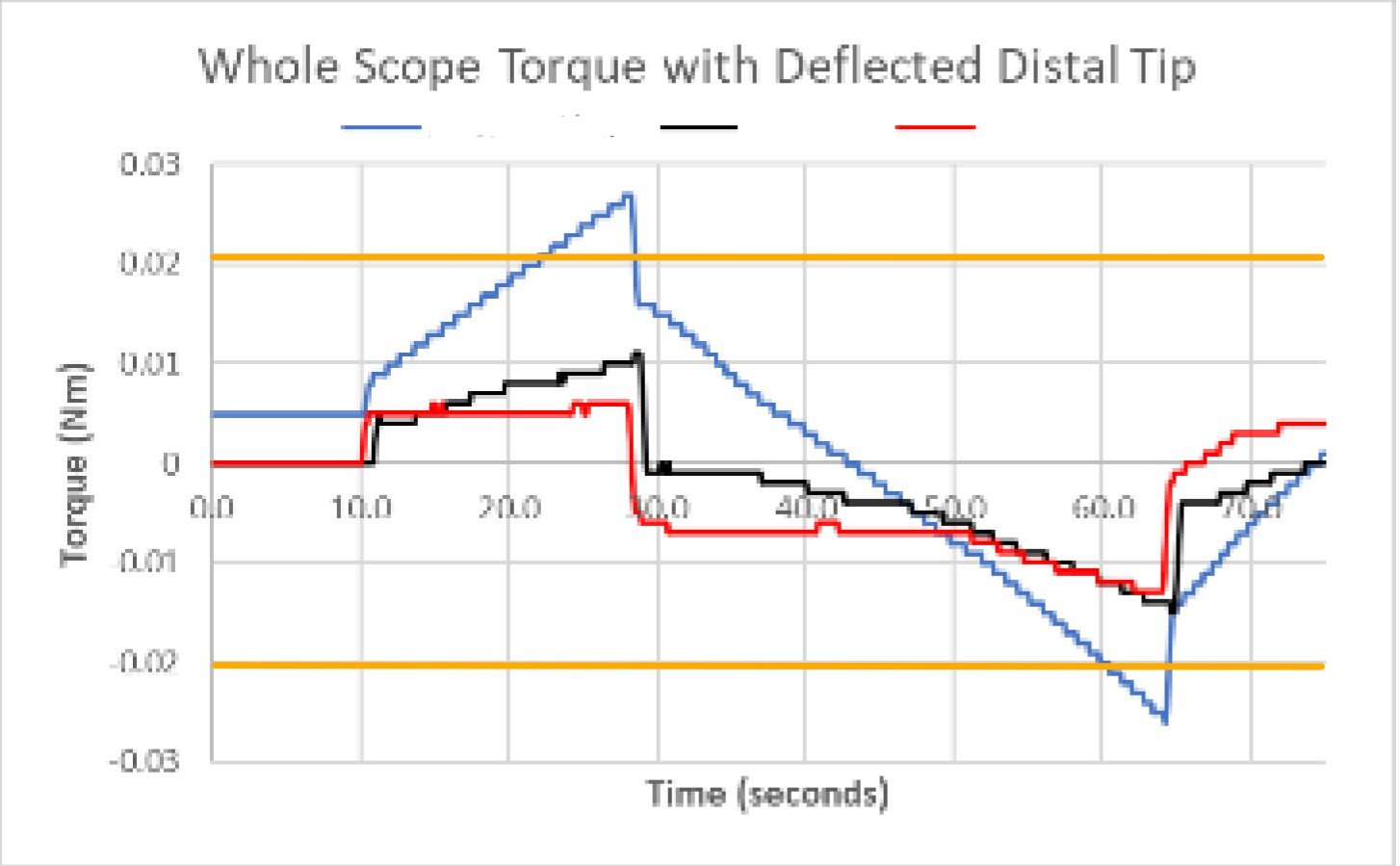
Example: For an articulating catheter assembly with low yield and extended cycle time, we investigated a conditioning process that required repeated actuation to meet functional requirements. Focused characterization identified localized material yielding in a specific region of the assembly that introduced variability during conditioning. By isolating this behavior and implementing a targeted conditioning step, the process became more consistent and predictable, reducing cycle time and improving yield without increasing process complexity.
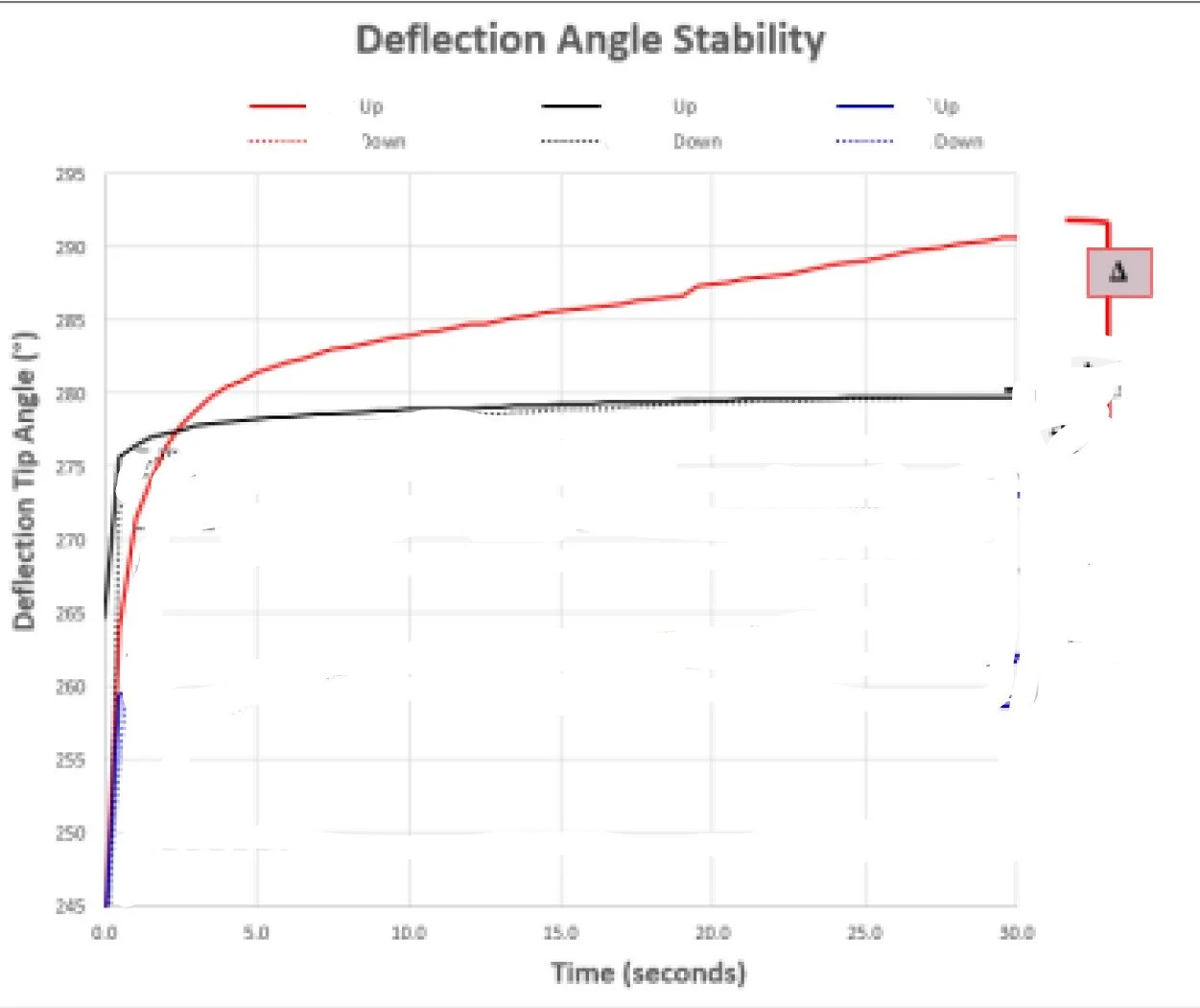
Effect of under tensioning of articulation stability
Insight gave optimized process window for tensioning to reduce scrap and operate within existing specifications.
Product Performance & Clinical Differentiation
Regulatory testing demonstrates safety and efficacy but rarely distinguishes performance between competing products, allowing devices with very different clinical behavior to appear equivalent on paper. This creates a gap between physician experience and objective data, making it difficult to quantify meaningful performance differences. We develop clinically relevant benchtop tests that quantify functional attributes physicians perceive but standard tests do not capture.
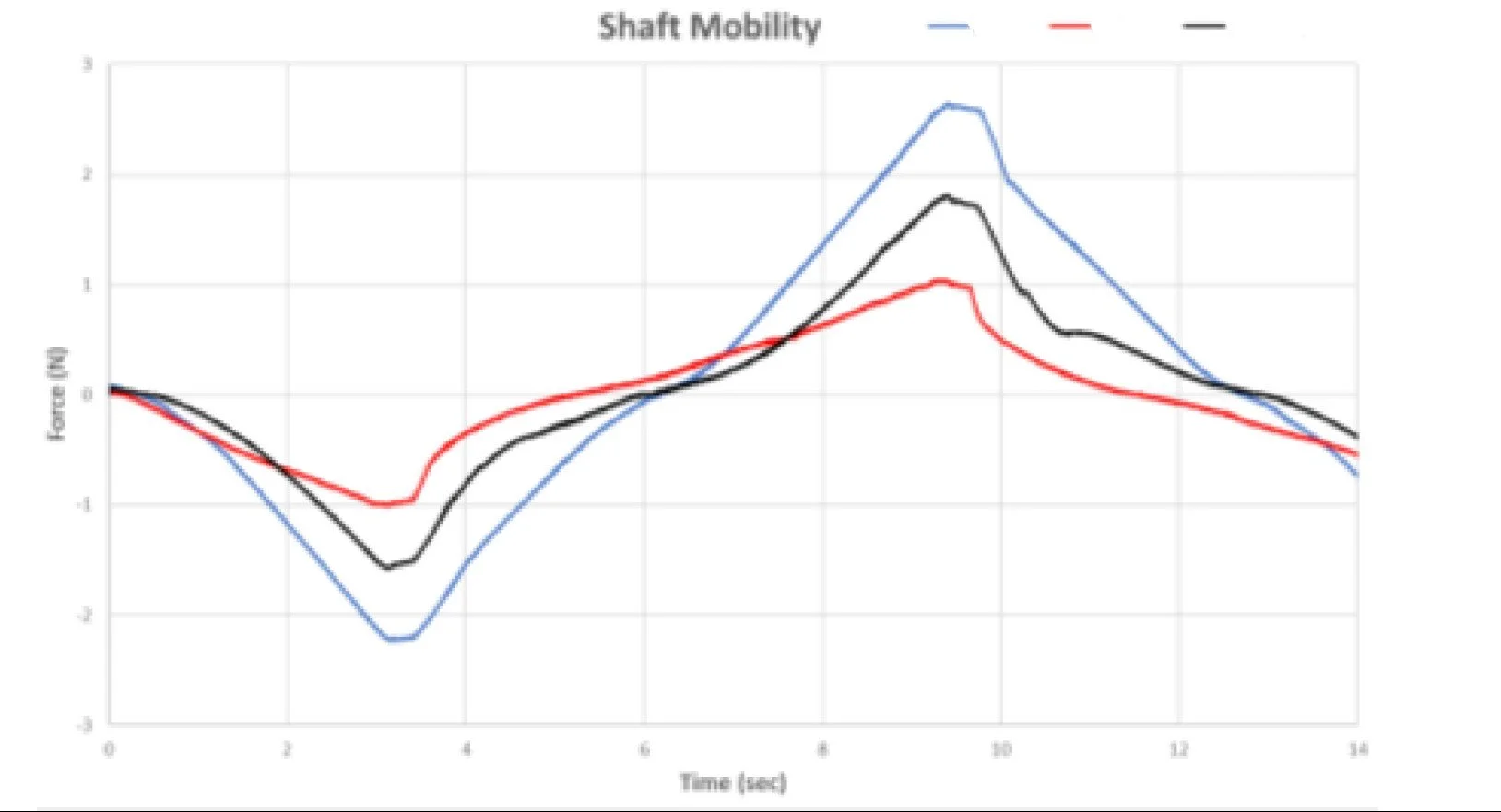
Example: In a study of multiple ureteroscopes, three products met identical dimensional and mechanical specifications yet exhibited noticeably different clinical behavior. Working with practicing urologists, we identified performance attributes related to articulation responsiveness, shaft stiffness transitions, and torque transmission that influenced maneuverability. We then developed benchtop tests to quantify these attributes under controlled conditions. The resulting data differentiated the devices in a way that aligned with physician preference and provided objective insight into clinically relevant performance differences.